Unlocking Medical Device Traceability with the Power of DPM Code Scanning
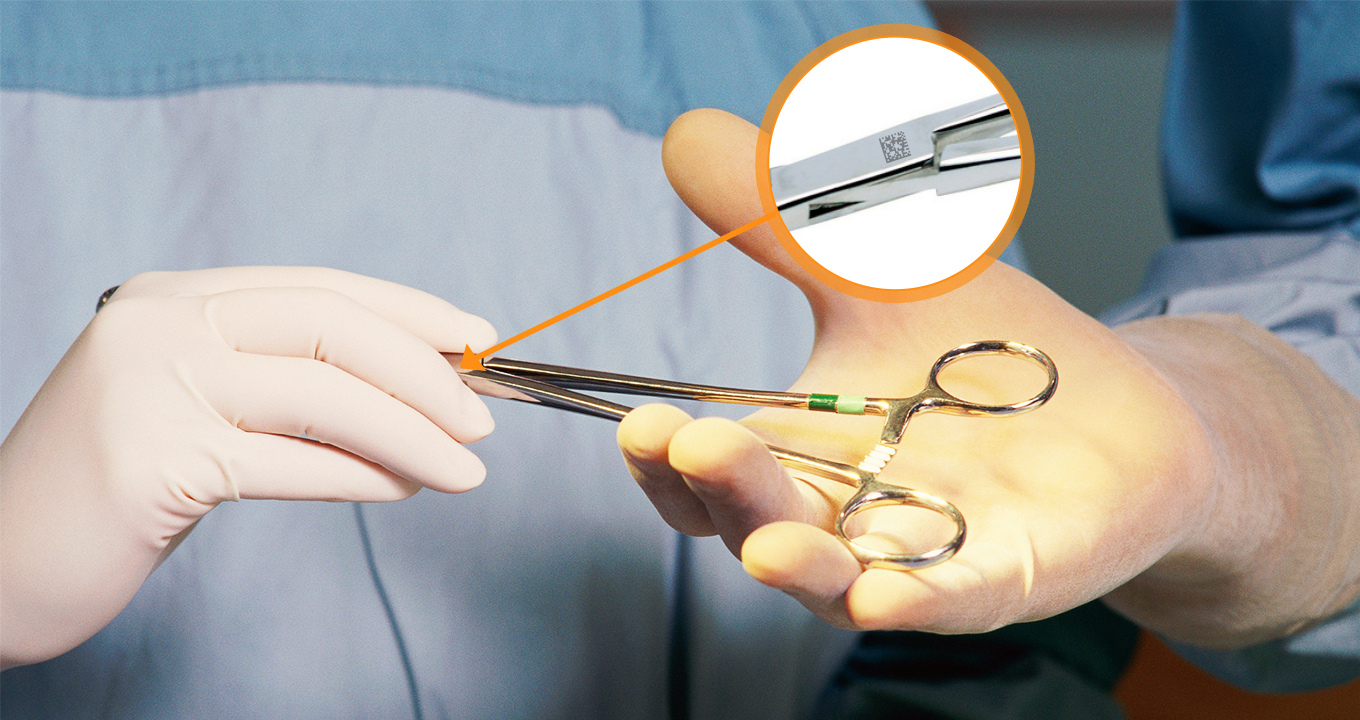
In today’s healthcare system, medical device traceability has become more critical than ever. To enhance traceability and safety in the modern healthcare landscape, UDI barcodes function as the standard for Unique Device Identification, providing a unique identifier for medical devices.
DataMatrix codes are frequently used for UDI and store a substantial amount of data in a compact, square matrix format, ensuring precise and efficient device tracking and traceability. This blog explains how DPM code scanning improves the traceability of UDI medical devices.
UDI Barcode Format: What Information Does It Contain?
Unique Device Identifier (UDI) is a unique code that typically includes alpha-numeric characters. The UDI barcode format can be further understood by taking a look at its constituents:
Device Identifier (DI): This segment of the UDI is obligatory and immutable, serving to distinguish the labeler from the precise version or model of the device.
Production Identifier (PI): It is a contingent and variable element of the UDI, which identifies one or more of the following aspects when incorporated on the device’s label. It includes:
- The expiration date of an individual device
- Serial number of an individual device
- The manufacturing date of an individual device
- Unique identification code mandated by §1271.290(c)
Embracing UDI Compliance with Ease
The Unique Device Identification (UDI) system was designed to enhance medical equipment tracking and traceability. Per regulatory guidelines, medical device manufacturers must affix their products with a Unique Device Identifier (UDI). This identifier encompasses crucial details, including the device’s lot number, serial number, and expiration date. When meeting UDI requirements, DPM codes are a reliable and effective option.
By implementing DPM code scanning, manufacturers can effortlessly acquire crucial data pertaining to every individual device, beginning from its inception and extending throughout its entire lifespan. This level of traceability helps in quickly recognizing and fixing any quality and safety issues, resulting in better patient outcomes and lower product recalls.
Tracking Dental Implants with Authenticity

Dental implants, known for their intricate designs and compact sizes, have historically been difficult to record and trace. DPM code scanning helps track various medical devices, including dental implants. Precision laser marking is used for engraving DPM codes onto dental implants, guaranteeing tiny yet highly legible DataMatrix codes for efficient traceability despite their small size and complex designs.
The durability and endurance of dental implants are critical for the well-being of patients. Leveraging DPM codes, dentists and surgeons may quickly and readily obtain vital information about an implant, including its production date, manufacturer, and quality control data, to make informed patient decisions.
Optimizing Traceability for Critical Components
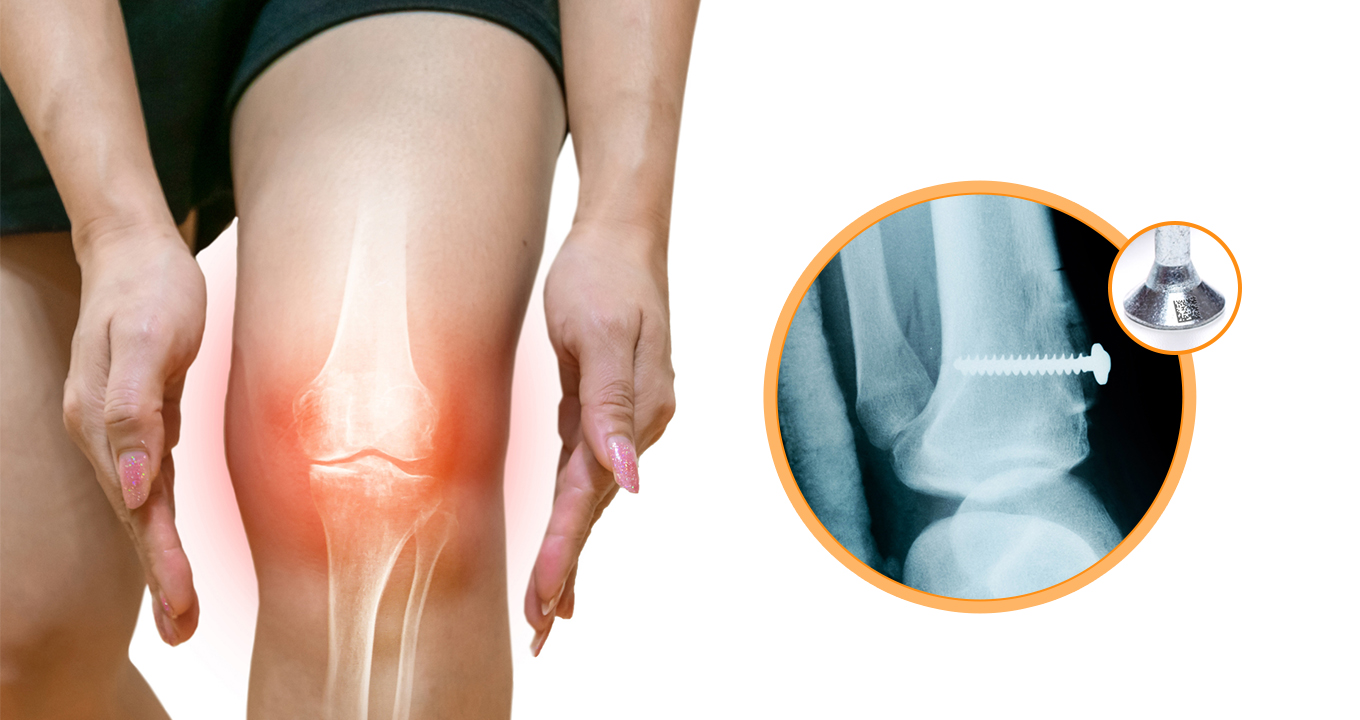
In medical technology, ensuring the authenticity and traceability of various complex and critical components is crucial for patient safety. DPM code scanning is essential for tracking the many components used in medical devices, ranging from catheters to other intricate diagnostic equipment, such as bone screws, arterial clamps, etc.
A catheter, for example, with DPM codes on its components enables medical professionals to immediately identify and replace any defective part, improving patient safety and lowering the chance of complications during treatments. The laser-marked UDI barcode, often a DataMatrix code, on these components can be easily scanned with a smart barcode scanner, allowing for easy monitoring, traceability, and better patient safety.
Innovative PEEK Solutions in Medical Technology
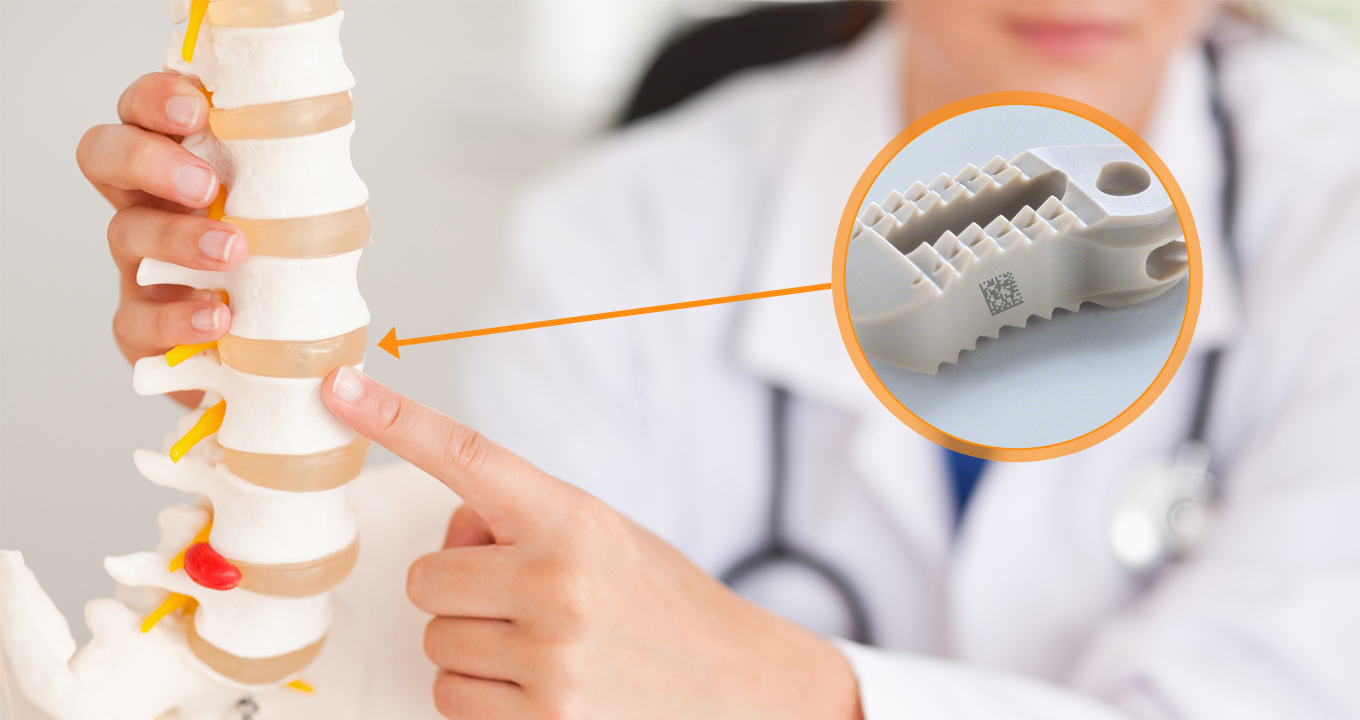
Polyether ether ketone (PEEK) is widely used in the medical field because of its high strength and biocompatibility. PEEK is utilized in various implantable medical devices, from spinal implants to dental prostheses.
To ensure traceability, DataMatrix codes in Direct Part Marking are engraved onto PEEK components to guarantee compliance with strict quality requirements and regulations. This traceability aids in establishing the validity of PEEK components, protecting patient health, and permitting product recalls as needed.
Transforming Traceability for Metal Implants
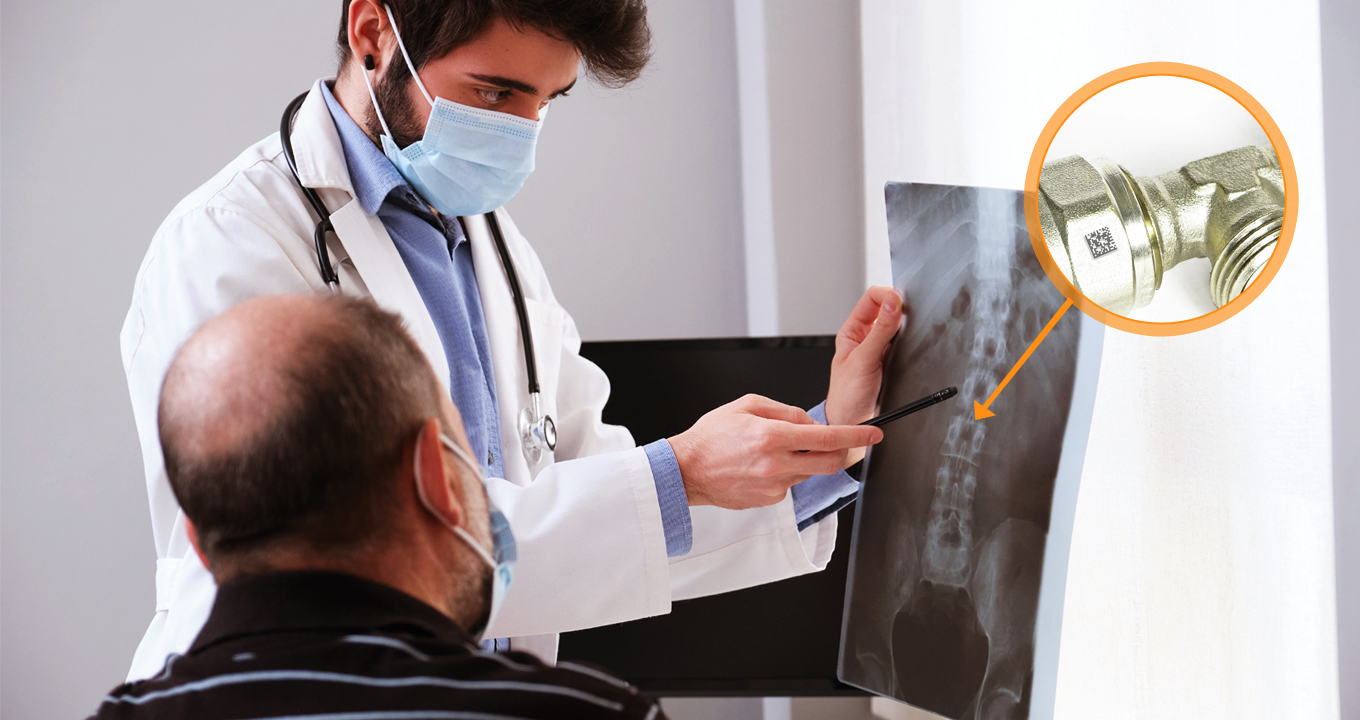
Metal implants are still an essential aspect of medical technology. Metal implants are necessary for many patients, from knee replacements to heart stents. DPM code scanning is also applicable to these devices.
Engraving DPM codes onto metal implants guarantees that they can be reliably traced, allowing healthcare providers to monitor their functioning and comply with the highest quality standards. In the case of a product recall or safety concern, the traceability of these implants is crucial in identifying and addressing the needs of impacted patients.
Common Challenges with Tracking UDI Barcodes and the Solution
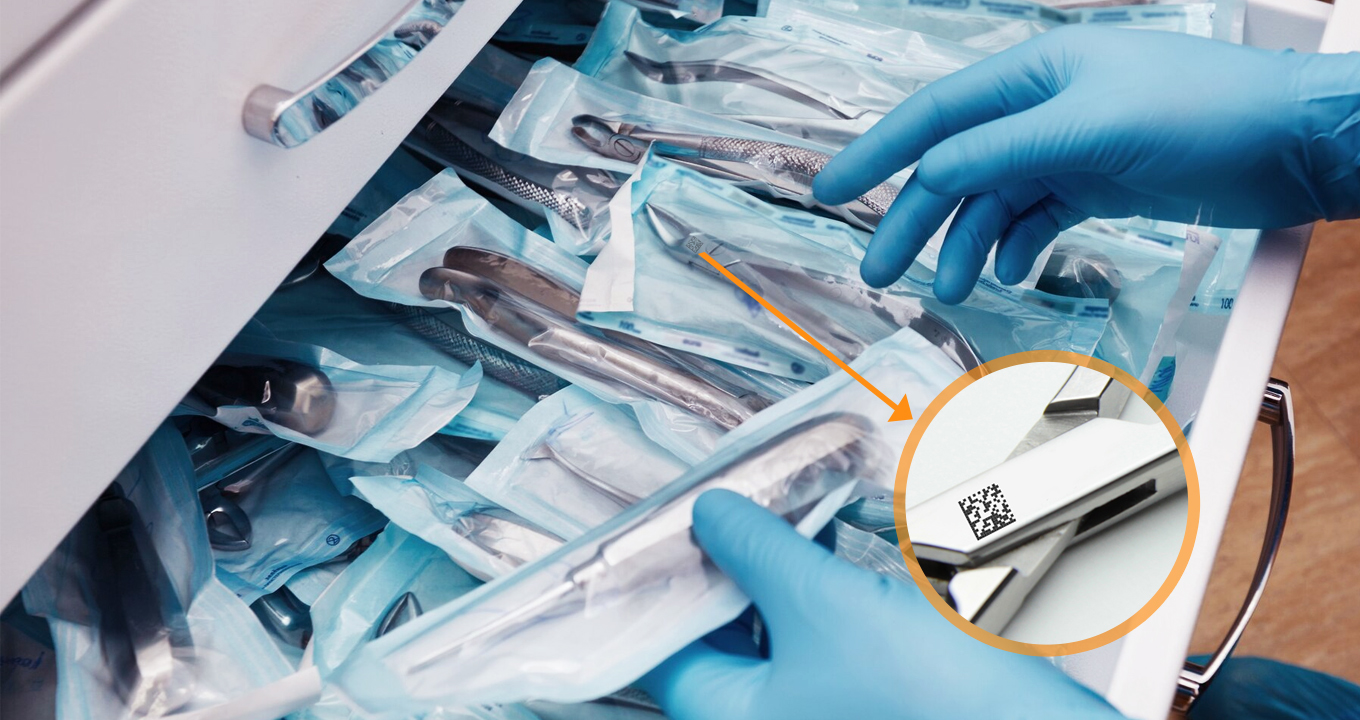
Tracking UDI barcodes is critical in the healthcare industry for ensuring patient safety, effective inventory management, and regulatory compliance. However, it is fraught with challenges. Dynamsoft Barcode Reader SDK acts as a robust UDI tracker, leveraging enterprise-grade barcode detection algorithms that excel even in the most challenging conditions.
Barcode Quality
Damaged or poorly printed DataMatrix codes can lead to reading errors, compromising patient safety. A regular UDI barcode scanner may fail to read such codes, but Dynamsoft Barcode Reader can read the toughest barcodes, even when poorly printed or damaged. This guarantees that data is reliably captured from various barcode qualities.
Barcode Standardization
Many healthcare organizations still use legacy systems for inventory and patient record-keeping. Integrating UDI barcode scanners with these preexisting systems can be difficult and expensive. However, seamless integration of UDI data into these systems is critical for effective tracking.
Dynamsoft Barcode Reader SDK supports a wide range of barcode formats and standards, enabling the seamless decoding of UDI barcodes regardless of the standard or format utilized. Not to mention, it offers flexibility to adapt to the requirements of various devices, critical for a UDI barcode scanner.
Integration with Legacy Systems
Legacy systems are still in use for inventory and patient record-keeping in many healthcare organizations. It can be difficult and expensive to integrate UDI barcode tracking with these preexisting systems. The seamless integration of UDI data into these systems is critical for effective tracking.
The seamless integration of the Dynamsoft Barcode Reader SDK with various software systems facilitates the adaptation to legacy systems. Its flexible APIs enable fast integration into new and preexisting healthcare environments.
Data Complexity
UDI barcodes frequently comprise substantial data, such as the expiration date, batch number, device identifier, and serial number. Managing this information and ensuring it is encoded accurately into the barcode can be difficult, particularly for large-scale manufacturers.
The capability of the Dynamsoft Barcode Reader SDK to extract structured data from barcodes facilitates the management of the intricate information contained within UDI barcodes. By granting access to the specific data elements, it facilitates enhanced integration with systems designed for inventory and monitoring.
Regulatory Compliance
UDI trackers are critical to ensure adherence to regulatory mandates, including those established by the FDA. It can be challenging to ensure that all devices are marked and traced per these regulations; failure to do so may result in legal and financial repercussions.
Assisting manufacturers and healthcare facilities in adhering to regulatory standards, DBR provides the precise decoding of UDI identifiers.
Poor Capture Rate due to Low-Quality Cameras
Another significant challenge in tracking UDI barcodes is the poor capture rate from low-quality cameras. In the healthcare industry, where barcode scanning is crucial for patient safety and compliance, Poor barcode scanning due to low-quality cameras can lead to unreadable barcodes, leading to unavailability of information during emergencies, or giving outdated information, which can subsequently affect patient’s treatment. Failure to decode the information through barcodes can be hazardous, especially in the healthcare industry, where accuracy is paramount.
UDI barcode scanners may need help to capture data accurately when faced with low-quality imaging. However, Dynamsoft Barcode Reader SDK addresses this issue by incorporating advanced algorithms that excel in challenging conditions. Its ability to read UDI barcodes efficiently, even with low-quality cameras, ensures the reliability of data capture.
Dynamsoft Barcode Reader SDK for Scanning UDI Barcodes
The assurance of medical equipment tracking is crucial in the rapidly developing field of healthcare technology. At Dynamsoft, we are proud to be at the forefront of this change as a leading provider of DPM code scanner SDKs, providing the industry with solutions that ensure not just patient safety but also commitment to regulatory norms.
Try our SDK to analyze its performance under various conditions. Contact our technical experts to see how our SDK caters to your use case.


 Blog
Blog