Traceability at Scale: How Batch Scanning Transforms Pharmaceutical Operations

The pharmaceutical industry stands at a critical inflection point. As global supply chains grow increasingly complex and regulatory frameworks tighten, the ability to track every unit, from the research lab to the pharmacy shelf, has become non-negotiable. A single scanning error can cascade into batch rejections, compliance violations, and patient safety risks.
For manufacturers, packaging facilities, and supply chain leaders, the focus has shifted from whether to implement traceability to how to achieve it at scale. Batch barcode scanning is transforming how the industry captures, verifies, and tracks products throughout their lifecycle.
Why Traceability Matters More Than Ever
The urgency of pharmaceutical traceability is driven by a regulatory landscape that is reshaping the industry.
The pharmaceutical landscape has undergone seismic shifts. Regulatory agencies worldwide have implemented stringent serialization mandates to combat counterfeit drugs and protect patients. In the United States, the Drug Supply Chain Security Act (DSCSA) mandates unit-level traceability with full enforcement through 2024-2025. Similarly, the European Union’s Falsified Medicines Directive (EU FMD) requires serialization at the point of dispensing.
These regulations reflect a commitment to patient safety. Comprehensive traceability systems can reduce medication errors by up to 56% and help protect against the $200 billion global counterfeit drug market.
Beyond compliance, traceability delivers operational efficiency:
- Faster batch release cycles through automated verification
- Reduced recall costs by precisely identifying affected inventory
- Improved forecasting with granular supply chain data
- Enhanced patient outcomes through error prevention
Traditional manual scanning cannot keep up with current production volumes or meet the strict accuracy standards required by regulators.
The Challenge of Pharma Traceability
Despite clear benefits, pharmaceutical companies encounter significant challenges when implementing traceability systems at scale.
Fragmented Systems and Data Silos
Modern pharmaceutical supply chains include many touchpoints, such as raw material intake, production, quality control, packaging, distribution, wholesalers, and pharmacies. Each often uses separate systems, including legacy MES, WMS, and LIMS, which struggle to communicate and create visibility gaps.
Volume and Velocity Pressures
Consider a typical packaging operation: hundreds of vials moving at high speed, each requiring verification of its 2D DataMatrix code, which contains the GTIN, serial number, lot, and expiration data. Manual scanning becomes an immediate bottleneck—operators cannot maintain accuracy when processing one unit per second in batches containing thousands of items.
A 2024 industry survey revealed 67% of pharmaceutical manufacturers identified “scanning throughput limitations” as their top operational challenge.
Common Pain Points
- Manual scanning bottlenecks: One-at-a-time processing creates throughput constraints.
- Misread and missed labels: Damaged labels, glare, and curved surfaces cause failures.
- Compliance documentation burden: Batch records require proof-of-scan for every unit.
- Operator fatigue: Repetitive tasks increase error rates.
- Integration complexity: Connecting hardware to enterprise systems requires significant IT resources.
Enter Batch Barcode Scanning: Traceability at Scale

Addressing these challenges requires technology that fundamentally changes how pharmaceutical products are scanned and tracked.
Batch barcode scanning enables the simultaneous capture and decoding of over 100 barcodes in a single image, rather than scanning each barcode individually.
How It Works
- Multi-frame image capture: High-resolution cameras capture wide-field images of product batches.
- AI-enhanced detection: Deep learning identifies barcodes despite occlusion, angles, curves, or lighting challenges.
- Panoramic stitching: Multiple frames combine for comprehensive coverage.
- Parallel decoding: All detected barcodes are decoded simultaneously using optimized algorithms.
- Validation and verification: Results are cross-referenced against expected values, flagging anomalies in real time.
- Data integration: Structured output feeds directly into MES, WMS, or quality systems with full audit trails.
The technology addresses real-world challenges, including reflective packaging, cylindrical vial surfaces, motion blur, and small or dense 2D codes.
Core Benefits
Accuracy: Advanced algorithms achieve 99.9%+ read rates, virtually eliminating missed scans that create compliance gaps.
Efficiency: Processing hundreds of codes in seconds transforms throughput. Operations requiring multiple operators can be handled by automated imaging stations.
Compliance: Built-in image archiving provides documented proof of scan, while structured data ensures compliance with GS1 standards and DSCSA data exchange formats.
Real-World Pharma Use Cases
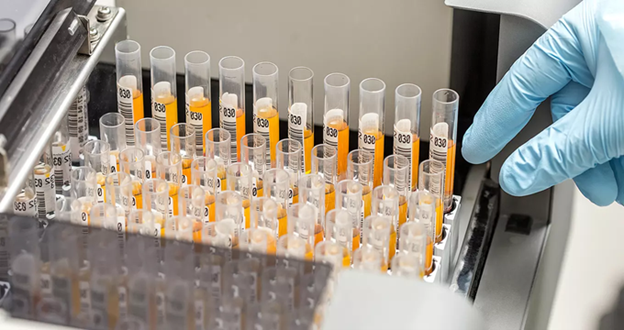
Batch scanning delivers measurable improvements across the pharmaceutical supply chain.
Manufacturing & Packaging Lines
Batch scanning systems mounted above conveyor belts provide:
- 100% serialization verification at line speed
- Immediate rejection of units with printing defects
- Automated aggregation of data linking units to cases to pallets
- Real-time batch record generation
Before: A mid-sized manufacturer required four operators to manually scan products, achieving ~80 units per minute with a 2-3% error rate. After: Automated batch scanning handled 400+ units per minute with 99.9% accuracy, reducing batch release time by 60%.
Warehouse & Distribution Centers
Batch scanning enables:
- Rapid intake verification: Scan entire pallets in under 30 seconds
- Automated exception flagging for missing or unauthorized products
- Outbound audit compliance before shipment
- Verification time outside cold storage is minimized.
Major distributors report a 70% reduction in receiving time while virtually eliminating trading partner chargebacks for data discrepancies.
Clinical Trials & Research Laboratories
Batch scanning streamlines:
- Kit preparation verification before shipment to trial sites
- Sample tracking: Rapidly catalog biological samples
- Temperature excursion documentation
- Audit trail generation for regulatory submissions
Returns & Recalls Management
Batch scanning allows companies to:
- Rapidly identify affected inventory across networks.
- Verify disposal of recalled units with documentary evidence.
- Isolate counterfeit infiltration by cross-referencing serial numbers.
- Generate regulatory reports with complete traceability chains.
Companies can now complete recall identification in hours instead of days, improving patient safety.
Accuracy and Compliance: Why It Matters

Meeting regulatory requirements isn’t just about avoiding penalties; it’s about building systems that protect patients and ensure product integrity.
GS1 Standards Foundation
The healthcare industry has converged on GS1 standards as the global language for product identification. GS1 DataMatrix codes encode GTIN, Serial Number, Lot/Batch Number, and Expiration Date. Batch scanning systems must reliably decode these standardized formats.
Regulatory Compliance Requirements
DSCSA (United States): The FDA requires pharmaceutical supply chain partners to electronically capture and exchange transaction information. By 2024-2025, this includes unit-level serialization and systems capable of product verification within 24-48 hours, both of which are impossible without reliable automated scanning.
EU FMD (European Union): Mandates unique identifiers on prescription medicine packaging, repository upload at packaging, verification at dispensing, and anti-tampering devices.
Audit Trail and Proof-of-Scan
Batch scanning systems provide:
- Timestamped image archives: Visual evidence of what was scanned and when
- Geolocation data and operator accountability
- Exception reports with disposition records.
- System validation documentation proving the accuracy specifications
This comprehensive documentation streamlines audit preparation, making it a straightforward process.
Technology Deep Dive
Technical decision-makers evaluating batch scanning solutions must understand the underlying technology to make informed choices.
AI-Enhanced Barcode Detection
Deep learning object detection identifies code locations regardless of orientation, partial occlusion, variable lighting, or non-planar surfaces. Dynamsoft’s barcode reading SDK incorporates AI-powered detection, increasing successful reads by up to 26.5% in challenging conditions.
Multi-Frame Analysis for 100% Capture Assurance
For critical pharmaceutical applications, 95% accuracy is insufficient because missed scans result in compliance failures. Multi-frame analysis captures multiple images, aggregates results, and flags unreadable positions for manual intervention, achieving near-perfect capture rates.
Deblurring and Image Enhancement
Motion deblurring algorithms sharpen images affected by movement, contrast enhancement improves low-quality prints, and glare reduction normalizes reflective surfaces. Dynamsoft Barcode Reader v11.2 delivers 44% faster processing while handling blurred and motion-affected codes.
Integration Architecture
- RESTful APIs for MES/WMS/LIMS integration
- Database connectors for SQL Server, Oracle, and PostgreSQL
- GS1 EPCIS event generation for supply chain visibility
- Cloud-ready: Deployable on-premises or in AWS/Azure
- SDK flexibility for JavaScript, Python, Java, C++, C#, and mobile platforms
The Business Impact
Beyond technical capabilities, pharmaceutical executives need to understand the tangible return on investment that batch scanning delivers.
Operational Efficiency Gains
Organizations implementing batch scanning report:
- 5-10x faster processing in receiving/shipping
- 50-70% reduction in operator scanning time
- 30-50% improvement in batch release cycle times
For a manufacturer releasing 100 batches per month, reducing release time by 2 hours per batch saves 200 hours per month, equivalent to 2.5 full-time employees.
Error Rate Reduction
Manual scanning error rates range from 0.5% to 3%. For a distribution center processing 1 million units per month, reducing errors from 1% to 0.01% eliminates 9,900 exceptions monthly and saves 1,650 to 4,950 labor hours.
Compliance Risk Mitigation
FDA warning letters for DSCSA violations can lead to operational disruptions and reputational damage. The cost of achieving compliance through reliable automation is negligible compared to enforcement consequences.
Total Cost of Ownership
Typical implementation investment ranges from $60,000 to $250,000. Annual labor savings are $150,000 to $500,000. Payback periods are 6 to 18 months, with a 3- to 5-year ROI of 300% to 500%.
Future Trends in Pharma Traceability
Pharmaceutical traceability will continue to evolve as emerging technologies further enhance supply chain visibility and patient safety.
Hybrid Tracking Technologies
- RFID integration for pallet-level aggregation combined with barcode precision.
- IoT sensor fusion providing condition monitoring alongside identity tracking.
- Blockchain creates immutable traceability records shared across supply chain partners.
AI-Driven Inspection and Quality Control
- Automated visual inspection detecting packaging defects during capture
- Predictive quality analytics identifying upstream manufacturing issues
- Intelligent exception handling with AI triaging anomalies
Unified Lifecycle Traceability
End-to-end visibility covers raw material sourcing, manufacturing, distribution, patient dispensing, and post-market surveillance. Batch scanning provides the foundational data infrastructure to achieve this goal.
Regulatory Evolution
Expect regulators to expand serialization requirements, mandate enhanced data sharing, require advanced analytics capabilities, and increase enforcement as grace periods expire.
How Dynamsoft Powers Traceability at Scale
When implementing batch scanning technology, choosing the right partner with proven pharmaceutical expertise makes all the difference.
Dynamsoft Barcode Reader SDK
Our flagship SDK delivers:
- Industry-leading accuracy: 99.9%+ read rates across 30+ barcode symbologies, including GS1 DataMatrix and Pharmacode
- Batch scanning capability: Decode 100+ barcodes simultaneously in one frame
- Challenging condition performance: Handles blur, glare, damage, curvature, and low-contrast printing
- Cross-platform support: Web, mobile (iOS/Android), desktop (Windows/Mac/Linux), and embedded systems
- Pharmaceutical-optimized: Native support for medical device barcodes (UDI), prescription drug codes (NDC), and serialization standards
Dynamsoft Batch Barcode Scanner
Engineered for zero-tolerance accuracy across regulated, high-volume scanning environments:
- Panoramic batch scanning with multi-frame stitching, capturing 100+ barcodes at once
- Real-time interactive visualization with cross-validation and missed-barcode highlighting
- Flexible deployment across iOS, Windows, Linux, and Web (beta), via APIs, containers, or embedded SDKs
- Compliance-ready with panoramic image archiving, barcode positional data, audit logging, and structured exports
Performance Improvements
Customers achieve over 99% first-pass read rates on damaged labels, with Sakura Finetek scanning 180 tissue cassettes in 20 seconds at 99.9% accuracy. Batch scanning processes 100+ items or 150 in under 2 minutes, saving up to 80% time versus individual scans. A 20% boost in speed and recognition applies to dense QR codes.
Key Features and Support
Our SDK supports pharma symbologies such as Pharmacode and GS1 DataBar, as well as image archiving for compliance tracking from production to shelf. Regular releases add decoding for drug labels and regulatory needs. Solutions enable lab positioning and GS1 parsing for supply chain traceability. Ready to Transform Your Operations?
See batch scanning in action: Try our interactive demo or explore our pharmaceutical healthcare solutions.
Speak with our experts: Contact us today to assess your traceability challenges.
Conclusion: Building Traceability for the Next Decade
Pharmaceutical traceability is now an immediate operational imperative, not a future consideration. Pharmaceutical traceability is essential for patient safety, regulatory compliance, and operational excellence. The industry now focuses on how to implement comprehensive traceability at the scale, accuracy, and speed required by modern operations.
Batch barcode scanning is a significant advancement, eliminating throughput bottlenecks, reducing error rates, and minimizing manual labor. The result is greater trust and compliance through improved accuracy and scalability.
As serialization mandates mature and enforcement increases, organizations that invest early in robust, scalable traceability infrastructure will gain a competitive advantage. Timely action is essential, as every scan is critical in pharmaceutical operations.



 Blog
Blog